+++ Christmas Break +++
Please note that due to our Christmas Break incoming biological material cannot be processed from 16 Dec 2024 to 7 Jan 2025. We kindly ask you to send material for identification or analyses no earlier than 8 Jan 2025.
Orders and requests received during Christmas Break will be stored in our system, but not processed further.
Mycoplasma Detection Services
We offer testing cell cultures for the presence of mycoplasmas as a service. The most sensitive assays for the mycoplasma detection in cell cultures are the broth-agar microbiological culture method and the polymerase chain reaction (PCR), which are both routinely applied for the quality control of the public cell line collection. Either mycoplasma test may be performed by us as a service.
Download our submission form.
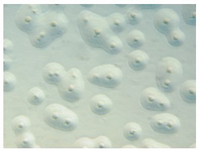
The broth-agar microbiological culture method uses cell culture supernatant containing some eukaryotic cells, which is inoculated to two different rich liquid media (Friis and PH). The cultures are then incubated for at least four days to enrich for mycoplasmas. Subsequently, aliquots of the suspensions are transferred to the respective agar plates. The plates are further incubated for two weeks or until morphologically typical mycoplasma colonies are visible by inverse microscopy. As can be seen from the description, the assay takes up to approximately two to three weeks (at least to obtain a negative result). An example of a positive agar plate is shown above.

The PCR assay is incomparably faster. In this assay cellular particles of the cell culture supernatant are isolated by centrifugation and the DNA is separated and purified using DNA extraction columns. The DNA extraction is important to eliminate Taq polymerase inhibitors which might be present in the cell cultures. Aliquots are used in PCR reactions with a primer mixture for the amplification of sequences which occur in the conserved 16S rDNA regions of almost all mycoplasma species listed so far in publicly available sequence databases. After contamination, a 504 - 519 bp DNA fragment (depending on the mycoplasma species) is visible in the ethidium bromide-stained agarose gel. As an internal control, we co-amplify a ca. 975 bp DNA fragment in a parallel to verify successful PCR amplification regarding sensitivity and absence of Taq polymerase inhibitors (example at right).
For information on how to send samples for mycoplasma testing, please refer to the mycoplasma section of our FAQ site
For pricing, please refer to our list.
For technical questions contact
Team Mycoplasma Detection |
Questions related to ordering
Team Sales |

